Natural killer (NK) cells have strong cancer immunotherapeutic potential that can be further augmented by engineering with a chimeric antigen receptor (CAR). CD38, a transmembrane glycoprotein involved in signal transduction and cellular adhesion, is highly expressed on the surface of the hematologic malignancies multiple myeloma (MM), acute myeloid leukemia (AML), Burkitt Lymphoma (BL) and T-cell acute lymphoblastic leukemia (T-ALL). FDA approved CD38 monoclonal antibodies (mAb) daratumumab and isatuximab are used to treat MM in patients, and have been tested to treat other CD38 expressing malignancies. NK cells play an imperative role in the efficacy of these antibodies and have natural antitumor activity against these cancers. However, because NK cells also highly express CD38, anti-CD38 mAb can cause NK cell fratricide, limiting their efficacy.
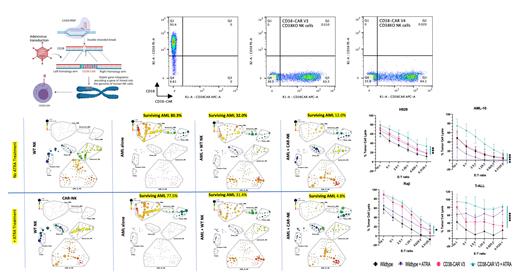
Here, we generated multiple CD38 scFv based on isatuximab sequences and generated fratricide-resistant primary CD38-CAR NK cells using CRISPR/Cas9 genome editing and AAV6 gene delivery. Using this approach, we inserted the CD38-CAR gene into the CD38 locus to efficiently generate simultaneous CD38 KO and CD38-CAR KI with a mean expression level of %60. The CAR-expressing NK cells could be expanded on mbIL21-expressing feeder cells to a clinically relevant number of cells with no decline in CAR-expression level during two weeks of expansion confirming the lack of CAR mediated fratricide.
We evaluated transduction efficiency and expression of CD38-CAR, and compared the cytotoxic function of two constructs with CD8 transmembrane (TM)/stalk and 41BB signaling domains and reverse heavy and light chains against AML, MM, T-ALL and BL immortalized cell lines and primary patient samples. We enhanced CD38 expression in target cancer cells by pre-treatment with all-trans retinoic acid (ATRA), a metabolite of vitamin A that pushes cells to maturation, consequently upregulating CD38. The sequential treatment of target cells with 10nM ATRA for 48 hours enhanced the cytotoxic activity of the CD38 KO/CD38-CAR-NK cells against primary MM, BL and AML samples. This would be particularly important for patients with low expression of CD38 on their cancers. The enhanced anti-tumor activity of the CAR was also studied and observed by CyTOF.
Additionally, to better understand the therapeutic potential of CD38-CAR NK cells compared to CD38-CAR T cells we generated CD38 KO/CD38-CAR T cells using the same engineering approach and comparing their cytotoxic function. We showed that both CD38-CAR-T and CD38-CAR-NK cells have comparable anti-tumor activity compare to their paired non-edited wild type cells.
Finally, we investigated the need for a CD38 KO to develop a fratricide-resistant therapy by comparing the cytolytic and metabolic functions of CD38 +/AAVS1 KO and CD38 KO CD38-CAR NK cells. We conclusively report a CD38-CAR NK cell therapy with enhanced metabolism and cytotoxicity toward a variety of hematologic malignancies, which can be further augmented by combination treatment with ATRA to target CD38-low malignancies.
Disclosures
Pereira:Kiadis Pharma, a Sanofi Corporation.: Patents & Royalties. Behbehani:Jazz Pharmaceuticals: Research Funding. Lee:Avidicure B.V.: Consultancy, Current equity holder in private company, Research Funding; Kiadis Pharma, a Sanofi Corporation: Consultancy, Patents & Royalties: licensed through Nationwide Children's Hospital. Naeimi Kararoudi:Kiadis / Sanofi: Consultancy, Patents & Royalties: Patents and Royalties.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal